What are IgM and IgG antibodies?
IgM is usually the first, specific antibody type generated by the body in response to infection. Your body usually generates these antibodies as its first line of defense against a foreign virus and can develop as soon as 3-5 days.
IgG antibody “is a protein that the body produces in the late stages of infection (possibly 21 days or greater) and may remain for up to months and possibly years after a person has recovered.”
IgM and IgG fight infections by targeting specific antigens on the surface of the SARS-nCoV-2 virus.
How is the Antibodies (Blood) test different from the Rt-qPCR,DNA (Swab) test?
The RT-qPCR,DNA test determines if the individual is infected with SARS-CoV-2 and considered to be able to transmit the disease (a positive test) or is negative for the virus. This test cannot tell whether a person is immune from past infection or has yet to be exposed and is still in danger.
The IgG & IgM antibodies test is based on the quantitative detection of IgM and IgG that are specifically generated by the body in response to SARS-CoV-2 infection. This can be used to determine recent & past exposure.
Researchers said having the IgG antibodies gives you protection from the virus -- but it doesn’t mean you’re immune for the rest of your life. You could get sick again. Still, they’re calling the test a milestone towards getting life back to normal.
Clinical Significance of Utilizing the DNA (swab) test along with the Antibodies (blood) test.
Antibody tests for COVID-19 cannot confirm the presence of the virus in your system at the present day. It can only tell whether you have been exposed in the past or if you have never been exposed to SARS-CoV-2. Consequently, it should only be used alone as a screening test and should be used in tandem with a genetic-based test to determine a complete status.
Clinical Significance (DNA + Antibodies)
| COVID-19 Real Time TR-qPCR(swab) | IgM Antibody (blood) | IgG Antibody (blood) | Clinical Significance |
|---|---|---|---|
| Not Detected | Not Detected | Not Detected | Patient was most likely not exposed to SAR-CoV-2 |
| Detected | Not Detected | Not Detected | Patient may be in window period of infection |
| Detected | Detected | Not Detected | Patient may be in early stage of infection |
| Detected | Not Detected | Detected | Patient may be in later or recurrent stage of infection |
| Detected | Detected | Detected | Patient is in active stage of infection |
| Not Detected | Detected | Detected | Patient may be in the reovery stage of infection |
| Not Detected | Detected | Not Detected | Patient may be in early stage of infection |
| Not Detected | Not Detected | Detected | Patient may have had past infection, recovered and possibly immune |
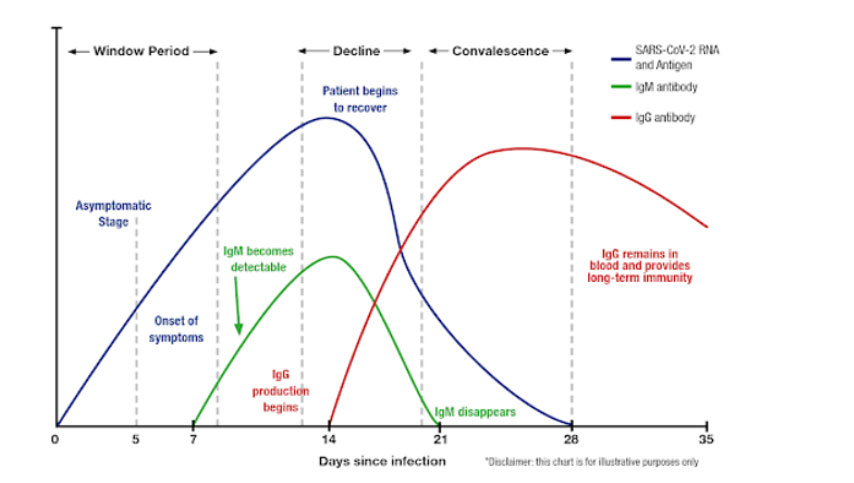
Variation of the Levels of SARS-CoV-2 RNA and Antigen, IgM and IgG after infection.
References:
- Weaver, C. Questions About Accuracy of Coronavirus Tests Sow Worry. The Wall Street Journal. April 2nd, 2020. Retrieved from https://www.wsj.com/articles/questions-about-accuracy-of-coronavirus-tests-sow-worry-11585836001
- Li R, Pei S, Chen B, Song Y, Zhang T, Yang W, Shaman J2. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science. 2020 Mar 16. pii: eabb3221.
- Lauer, S. et al., 2020. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Annals of Internal Medicine.
- National Health Commission of the People’s Republic of China, New Coronavirus Pneumonia Diagnosis and Treatment Program (Trial Version 7).
- To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC et al. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23. pii: S1473-3099(20)30196-1.
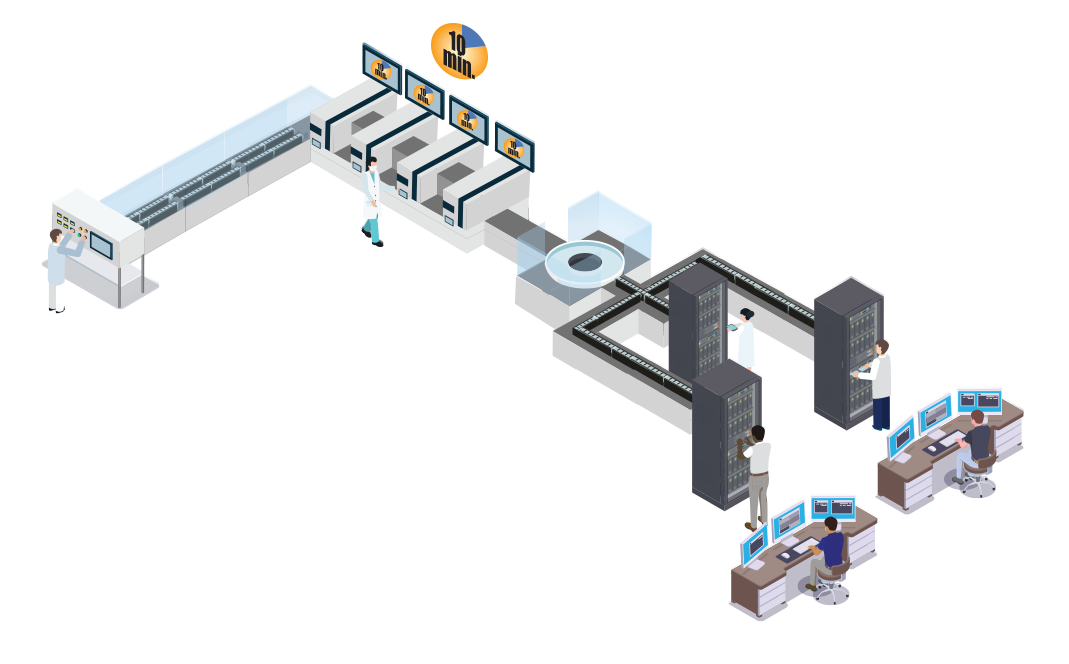
Cartridge Test Kits with results in 10 minutes
Cartridge based antibody tests only provide a postive or negative result for IgM and IgG. Some also do not have high clinical Sensitivity levels.

Our  COVID-19 Antibodies Test
COVID-19 Antibodies Test
Our Total IgG & IgM testing is performed in our CLIA High Complexity specialty lab on dedicated, automated instrumentation which is Authorized by the FDA (EUA).
Clinical Specificity: 100%
Clinical Sensitivity: 99.80%
We provide a Quantitative report if the Total Antibodies is Positive,which not only informs of detection, but also quantifies the concentration of antibodies detected.
Our Quantitative IgG & IgM
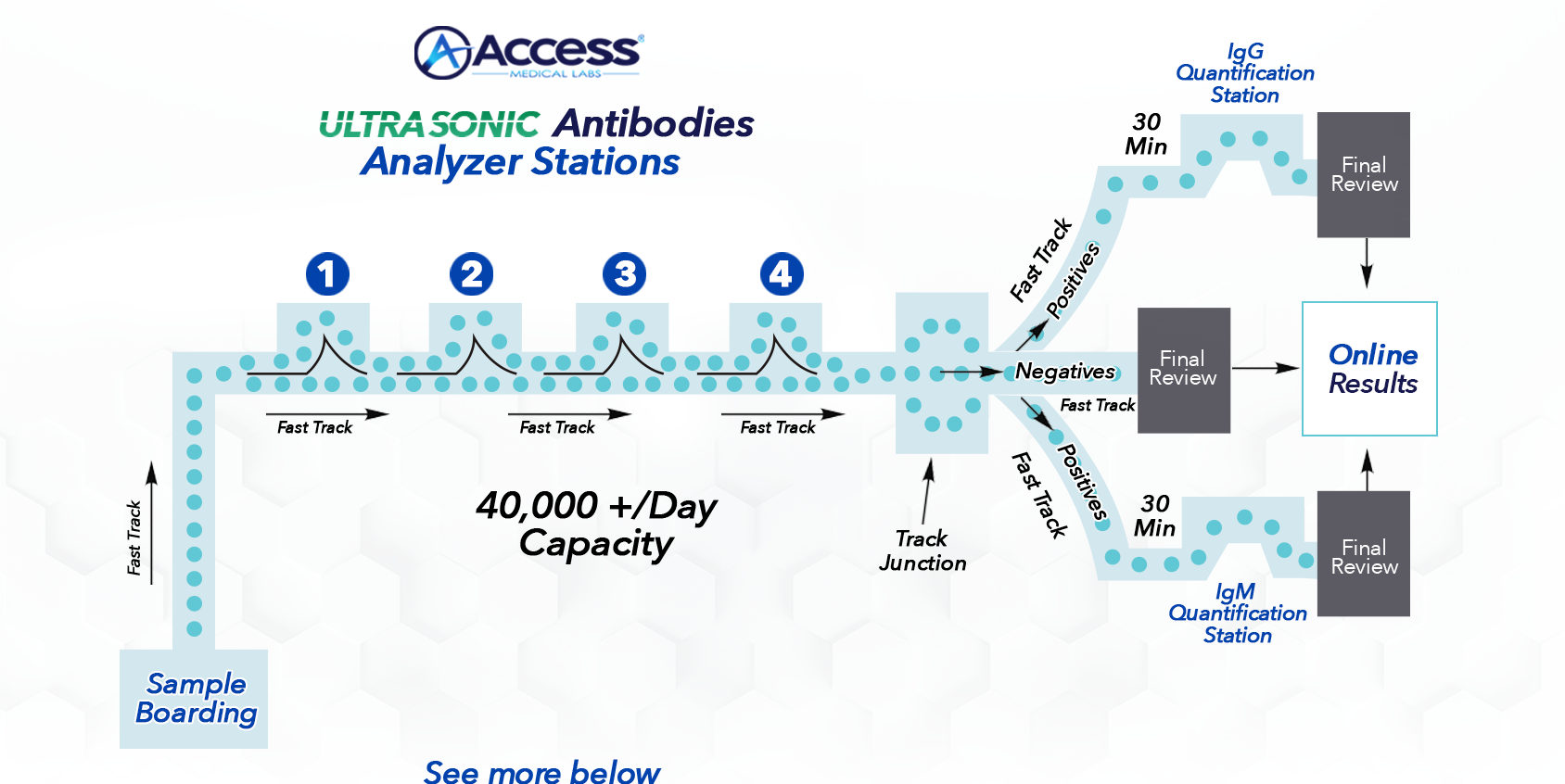
When a Total Antibodies test is positive, the sample will be sent to the IgG & IgM Quantification stations using our Fast Track Technology to identify which antibody(s) is positive and what the concentration level is.
Clinical Specificity: 95.60%
Clinical Sensitivity: 97.50%
The Key To getting Americans Back To Work
Scaling up COVID-19 IgG & IgM testing will help piece together a clear picture of how many Americans may have already been infected, built immunity and can get back to work. This is critical to re-opening the economy, and Access is here to help, one test at a time.
"Serological tests will enable us to determine what percentage of the population has been exposed to the virus," says Joanne Bartkus, PhD, director of the Minnesota Department of Health's (MDH's) Public Health Laboratory Division. "If we understand or find that a certain level of antibody does confer immunity to further infection, well, then that can be used to determine maybe who can go back to work, or who is less likely to be able to transmit the virus." - Joanne Bartkus, PhD.
- 1 Specimen: Serum
- 2 Temperature: Ambient
- 3 T/A: 24 hrs
- 4 Method: Chemiluminescence Immunoassay
- 5 Order Code: C19T