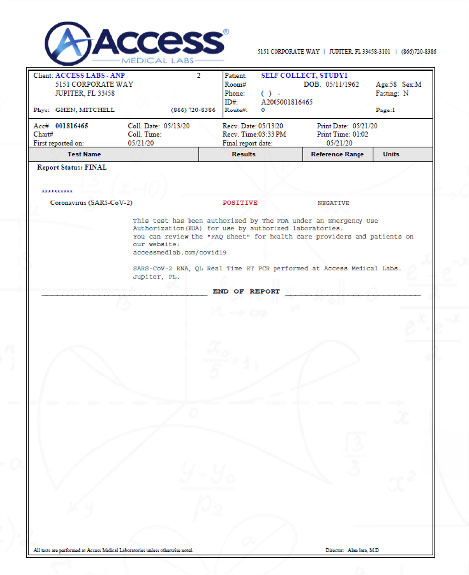
PCR TESTING WOULD NOT EXIST TO DETECT SARS-CoV-2.

The Inspiration for the Peace of Mind Experience.
Today we hear a lot of buzz around Rapid tests that give you results in 10 minutes or less without an explanation on how they differ from RT-PCR.
PCR testing is by far the most fascinating method I have ever seen and I stand behind it. In 1993 the PCR method won a noble prize, and in 2020 it stands the test of time.
It is the Gold Standard in DNA testing and for identifying the RNA/DNA of SARS-CoV-2 (COVID-19). We at Access wanted to create a service where you get the highest quality test, world class customer service and a rapid turn-around time for results. Our COVID-19 test using RT-PCR methodology is ran in our high-tech, state-of-the-art laboratory with results reported within 24hrs.
This was the inspiration for writing a step by step visual read on how your swab sample is processed to get a result within 24hrs.
Please enjoy this read and welcome to your personal laboratory.
We don’t just deliver results, we provide peace of mind®
The Method
The Lab Method is our blueprint for success when wanting to perform a reliable test for the detection of SARS-CoV-2. Excellent laboratories need immaculate lab methods. This takes endless amounts of research, development and troubleshooting to see if we can actually replicate what we say works. Can we say with great confidence that we have an excellent lab method?
Validation – The Confidence Level
The next step is validation. Validation is taking at least 30 positives and 30 negatives samples to see if we can use our method to receive the results that we claim are precise, sensitive, and specific to SARS-CoV-2. We dilute those samples and go to low detection levels three times, this is called the LoD (Limit of Detection) or what we at Access like to call "The Confidence Level." Can we do this at 2x and 3x and get reliable results? Are we confident that we can replicate the results? If so, we have a Validated Method that we can now use to run the COVID-19 test.

What is Sensitivity and what is Specificity?
Sensitivity and Specificity determine how well the test performs.
Sensitivity is how well the test can detect what it is looking for (SARS-CoV-2). The higher the sensitivity, the more you avoid the False Negatives.
Specificity is how specific the test is in only detecting what we are looking for (SARS-CoV-2) without something else interfering with the sample. Without a very specific test, you can run into False Positives.
The Access COVID-19 test is 94% Sensitive and 100% Specific.
Quality Assurance
The right method is key but without Quality Assurance the integrity of the specimen could be jeopardized. Every day, swabs arrive from all over the United States. Swab samples are received in the shipping department where they must be sorted, opened, checked to ensure the swab is secure in the transport tube and that the Kit ID card matches the sample. Next, the samples are moved to Data Entry. This area is responsible for entering patient data into our Laboratory Information System and barcoding of the samples takes place for track¬ing and reporting. The samples are then ready to move to the Molecular Lab where all the magic happens.
Our Talented Medical Technologists
The right talent is crucial because of the manual steps performed. Our Medical Technologists are responsible for thousands of patient’s samples daily. We need to secure the human gene sample out of your swab. Using precision robotic technology to pipette micro-samples of each patient's swab specimen, chemicals, and reagents into a small 96 well plate (see image below) is necessary to avoid contamination. Since we are dealing with Molecular analysis for this test, samples are brought down to microliters. For example, 5ul (microliters) size, is 0.005mL.
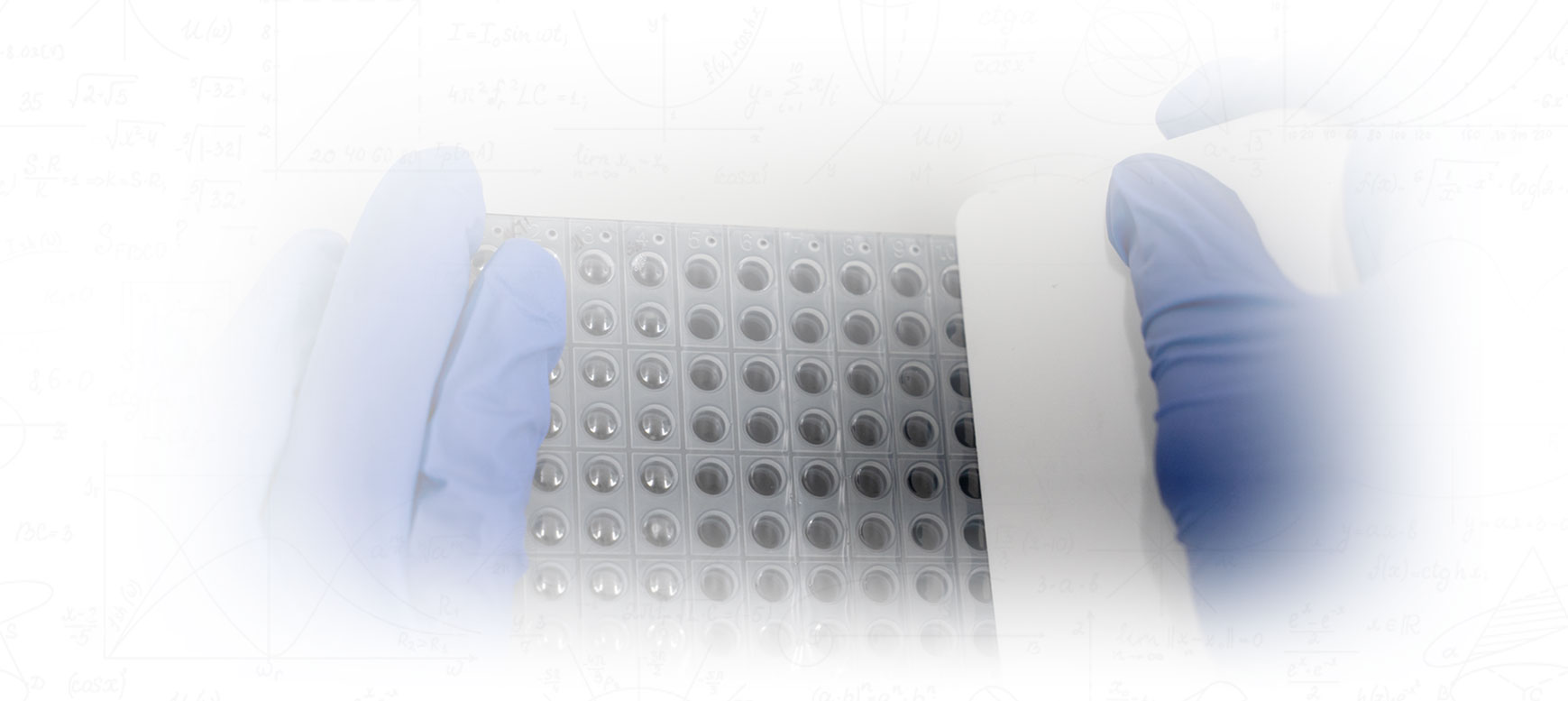
The PCR Set up
After the extraction process, we are going into PCR set up. We are taking the samples and bringing them down to the final molecular level which is precisely 5ul (microliters). The samples are pipetted into a PCR micro size 96-well plate. The optical film is carefully placed over the plate by hand. The film is then carefully sealed tight around each well to prevent evaporation, cross-contamination and to ensure the camera gets an accurate reading of each sample. The PCR plate then enters the PCR Studio.

Thermo Cycling Studios
It is time to head to our Studios. These thermocyclers are responsible for amplifying the DNA. There are three goals in Thermo cycling.
- Denaturing: Heating up the double-stranded DNA template which will separate the DNA into two single strands.
- Annealing: Lowering the temperature to enable DNA primers to attach to each strand.
- Extending: A new identical DNA strand is made here by the Taq polymerase enzyme.
- The process is repeated at every cycle leading to an exponential increase in the amplification.
The Taq polymerase enzyme is used to amplify the DNA.
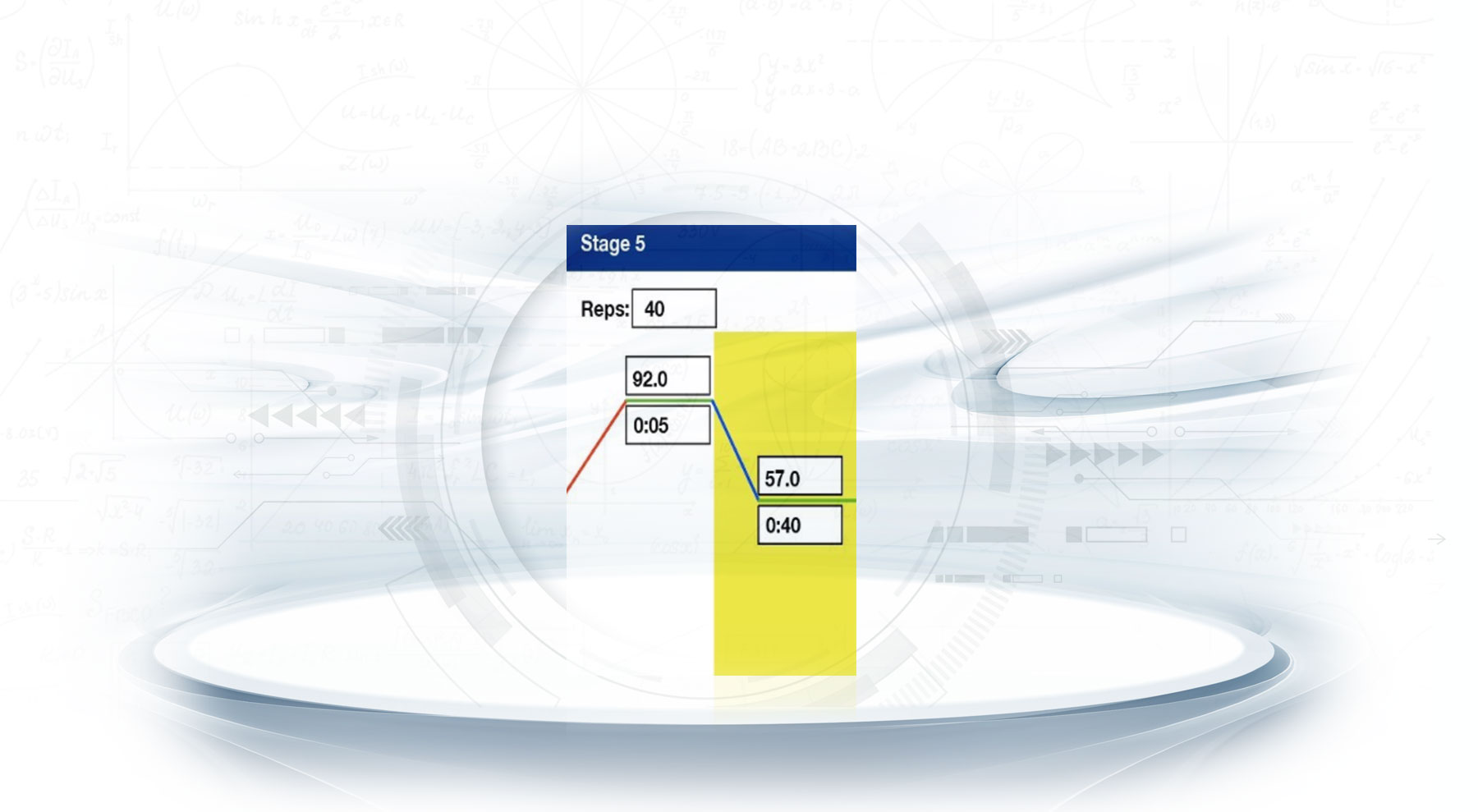
There are 5 stages in the SARS-CoV-2 method. We constantly cycle by bringing up the temperature of the sample UP and then dropping it DOWN. Look at it as building up the DNA and breaking it down to get a reaction. That’s essentially what’s happening while we multiply it. Stage 5 is where the sample is read in real-time.
Reading in Real Time
This is where we actually read the reactions from the thermocycler using a CCD camera, lighting, mirrors, and high-quality optical film. The Thermo Cycler is looking for a certain dye, which is a fluorescent reaction in the sample to read the presence of SARS-CoV-2. It does this by cycling the samples. We heat up to 92 degrees for 5 seconds then drop it down to 57 degrees for 40 seconds. When we are at 57 degrees for 40sec, that is when we are reading the sample. Then we cycle again. Stage 5 is 40 repetitions of this.
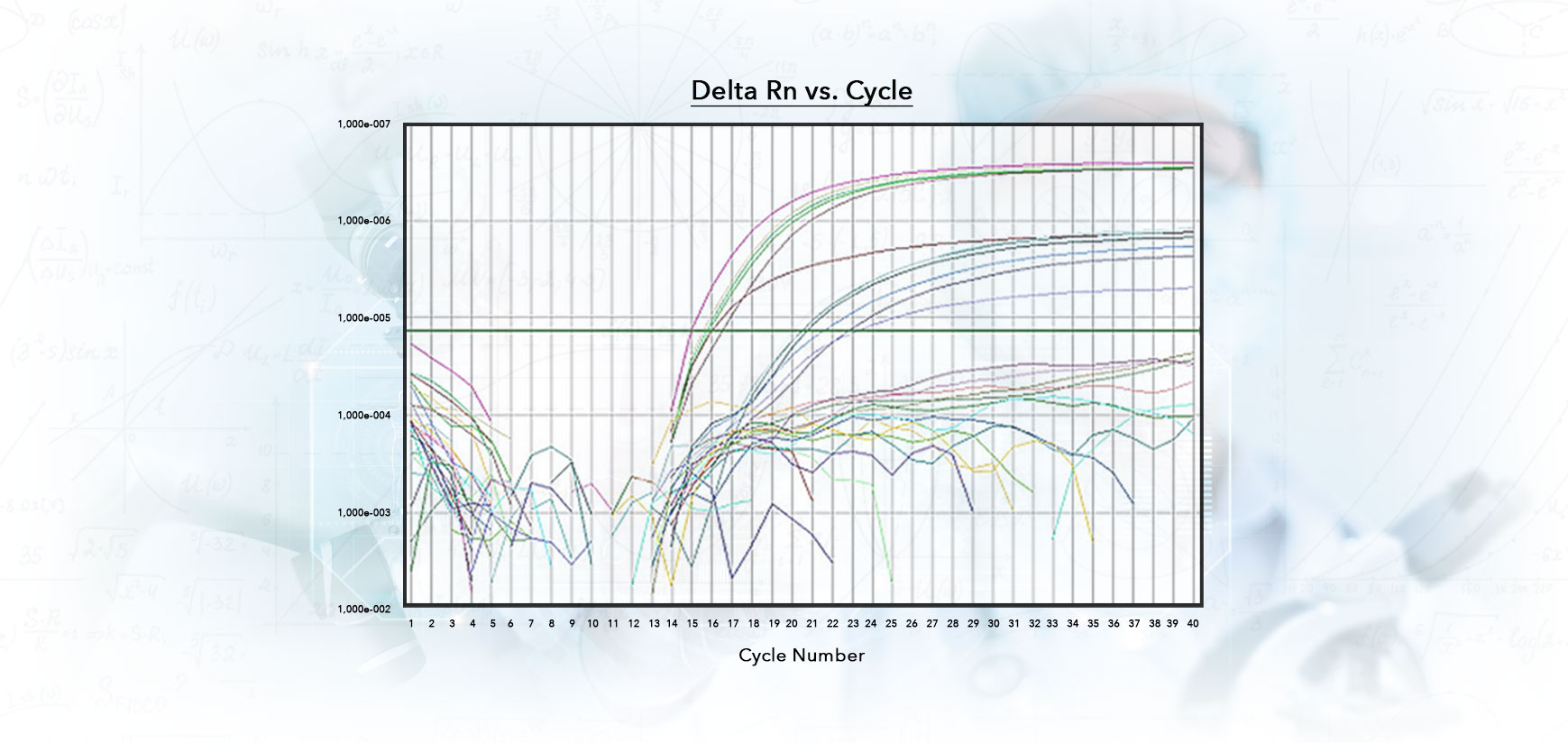
Examination
Each patient sample is carefully examined. The Tech carefully goes through each patient's amplification plot and determines the result. What the tech sees is how the sample responded to the chemicals and reagent that creates a dye,this is a fluorescent color signal that is recorded in real-time by the camera. Based on the strength of the signal, it will show up on an amplification plot for the tech to read. If there is no amplification of the sample, or it is below the set threshold, then the result will be Negative.
Approval
It is time to approve the results and digitally package them. The Digital Report is ready to leave our Laboratory Information System and head to your inbox via an Encrypted HIPAA compliant email.
We don’t just deliver results, we provide peace of mind®
All rights reserved 2020.